Elemental Analysis using XRF
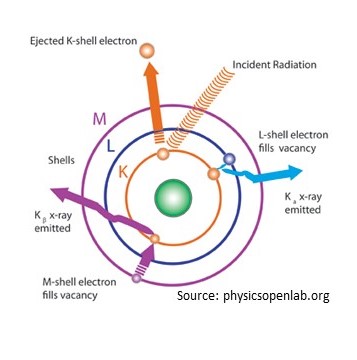
Find out what elements are in your samples at the Nebraska Center for Materials and Nanoscience (NCMN) on the UNL campus. Students are able to meet with scientists to determine the elemental composition. The XRF uses a palladium source of radiation to excite the electrons and measures the energy emitted as electrons move between valences. This transition energy is special for each element. This method can detect elements between fluorine and uranium on the periodic table.
Research Question:
- How will metals content of soil affect the metals found in vegetables?
- Students are encouraged to develop their own hypothesis if they are interested in a different topic.
Students will:
- Collect samples and send them to the NCMN for preparation
- Meet with scientists either in-person or online to process their samples and discuss the results
Lab skills learned:
- Sample collection and preparation
- How does XRF work
- What is Bragg's Law
Elemental Analysis Using XRF
COOKIE USAGE:
The University of Nebraska System uses cookies to give you the best online experience. By clicking "I Agree" and/or continuing to use this website without adjusting your browser settings, you accept the use of cookies.

